During the COVID-19 pandemic, mathematical modelling played a major role in informing public health policy responses. A key question for public health policy makers is whether the introduction of a virus into a population is likely to lead to sustained transmission. This is critical for understanding the epidemic and/or pandemic potential of a novel virus – notably, for example, following the first detected COVID-19 cases in Wuhan, China. Similarly, once a virus has become established within a country, understanding the risk of localised outbreaks occurring within sub-populations (e.g. in schools, workplaces and care homes) remains important for optimising ongoing public health measures.
In a recently published study in PNAS Oxford Mathematicians William Hart (pictured) and Robin Thompson, together with collaborators in Japan and South Korea, developed a multi-scale mathematical modelling framework for estimating the outbreak risk (the probability that an introduced case leads to sustained transmission) for a viral pathogen. Specifically, they derived the outbreak risk analytically under a branching process model of transmission (the population-scale model), accounting for changes in viral shedding during each infection (characterised by an individual-scale model). This is the first time that individual-scale viral dynamics have been included in outbreak risk calculations.
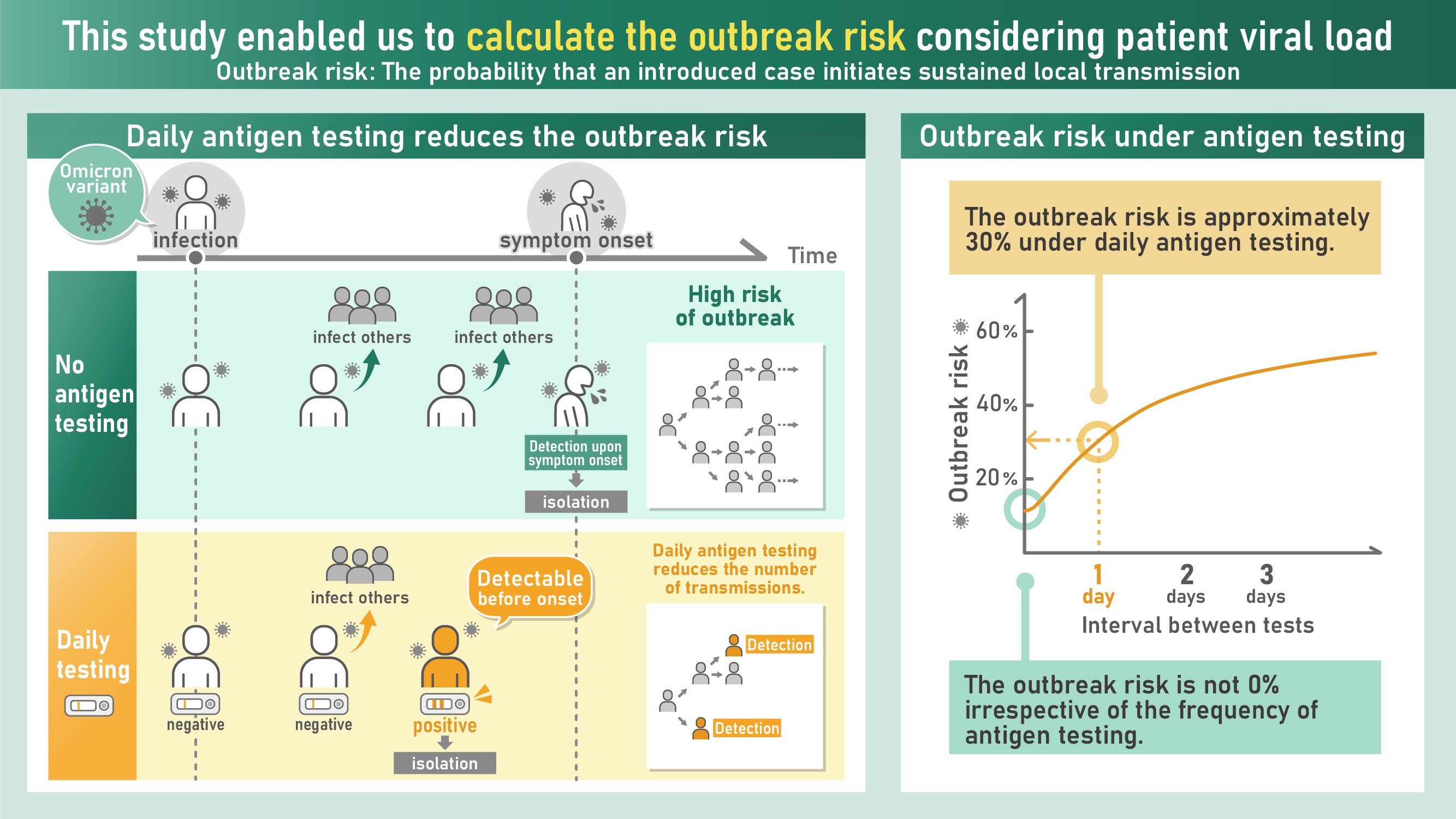
Individual-scale viral dynamics are crucial in determining the impact of numerous control interventions, for example mass lateral flow testing. A multi-scale modelling approach is therefore particularly useful for planning such interventions. To demonstrate this, the researchers considered the risk of localised COVID-19 outbreaks, and explored the effectiveness for mitigating this risk of regular antigen testing of the entire local population (i.e. routine lateral flow testing, as was implemented in the UK in the acute phase of the COVID-19 pandemic). In their baseline analysis, the researchers found that antigen testing can reduce the outbreak risk, but not prevent outbreaks entirely. However, the effectiveness of antigen testing depends on a range of factors, such as the extent of asymptomatic transmission, that are likely to differ between SARS-CoV-2 variants (and different viruses more generally), time periods and populations.
The COVID-19 pandemic has highlighted that infectious disease outbreaks remain a huge threat. The multi-scale modelling framework developed in this research can be used for guiding pre-emptive control interventions targeting a range of viruses going forwards.
William Hart is a Postdoctoral Research Associate in Climate Sensitive Infectious Disease Modelling, Wolfson Centre for Mathematical Biology.
Robin Thompson is Associate Professor of Mathematical Epidemiology and a fellow of St Hilda’s College.


