Have you ever wished that the battery on your phone would last longer? That you could charge it up more rapidly? Maybe you have thought about buying an electric vehicle, but were filled with range anxiety – the overwhelming fear that the battery will run out before you reach your destination, leaving you stranded? Oxford Mathematicians are hard at work demonstrating that mathematics may provide the key to help tackle problems faced by the battery industry. Robert Timms talks about the battery research going on in Oxford.
"There is a long history of battery research at Oxford: this month Professor John B Goodenough received the Nobel Prize in Chemistry for his work at Oxford University that made possible the development of lithium-ion batteries. His identification and development of Lithium Cobalt Oxide as a cathode material paved the way for the rechargeable devices such as smartphones, laptops and tablets that are now ubiquitous in today’s society. Given that Oxford can be viewed as the birthplace of rechargeable lithium-ion batteries, it is natural that the Oxford Mathematical Institute, with its long association with doing industrial mathematics, is now home to a vibrant battery research community focussed on the mathematical modelling of batteries.
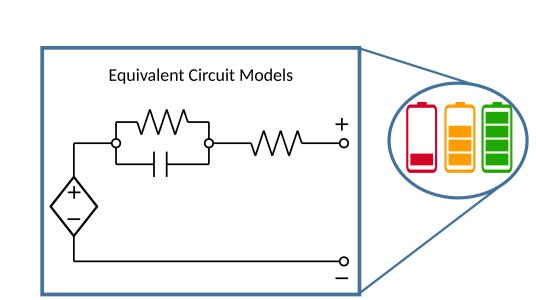
Mathematical models of batteries can be broadly categorised into two groups: equivalent circuit models, which make analogies with traditional circuit components such as resistors and capacitors; and electrochemical models, which describe the physical processes of mass and charge transport within the cell. Equivalent circuit models can be solved rapidly on cheap computing hardware, making them the ideal choice for real-time battery management applications. However, they provide limited physical insight into battery behaviour. On the other hand, electrochemical models are computationally expensive, but provide a much more detailed description of the internal physics of battery operation which can be used for improving cell design.
Figure 1: Equivalent circuit models describe battery behaviour using standard circuit components, and are used in real-time applications such as estimating State of Charge (how much battery life you have left). They are easy to interpret and computationally cheap to solve, but offer limited physical insight.
In order to develop electrochemical models that describe the physical processes that underpin battery operation it is necessary to account for effects that vary over length scales on the order of microns – this is similar to the breadth of a human hair! However, understanding how batteries operate as part of a device requires modelling on the length scale of centimetres. In order to bridge the length scale gap, Oxford Mathematicians use a technique called homogenisation which allows the description of the physics at the microscale to be systematically upscaled into effective equations on the macroscale.
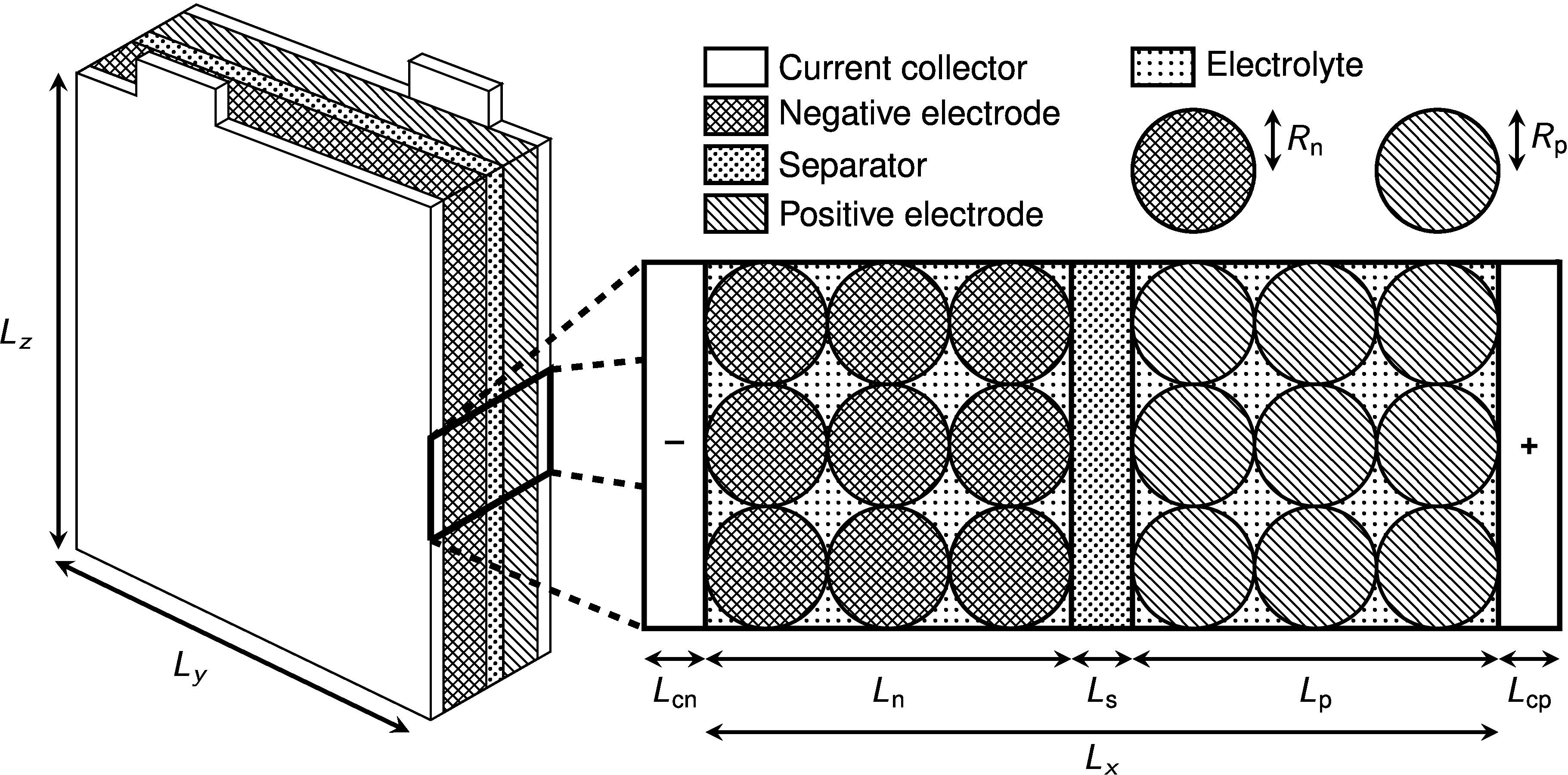
Figure 2: A typical single-layer pouch cell design (not to scale). Lithium-ion batteries are made up of a number of components: a negative current collector, porous negative electrode, separator, porous positive electrode and a positive current collector. The porous electrodes are made up of solid particles of spheres that can be modelled whose radius is of the order of tens of microns (much smaller than shown in this sketch). Physics processes on the particle scale must be upscaled to give effective equations for the behaviour of the cell as a whole. The dimensions of the width and height of the pouch cell, labelled here as Ly and Lz , are of the order of tens of centimetres.
Even after the electrochemical models have been upscaled to the cell level they still comprise a large collection of partial differential equations, so can be computationally expensive to solve and difficult to interpret directly. Starting with complicated electrochemical models and exploiting techniques such as asymptotic analysis, we systematically derive simplified physics-based models, which provide a useful theoretical middle ground between electrochemical and equivalent circuit models to support battery management, on-line diagnostics, and cell design. Using these simplified models we can better understand the underlying principles of battery operation and help to inform the design of new and improved lithium-ion batteries.
So, next time you are using your phone, think about all of the interesting mathematics being used to make your battery last longer."
Notes:
Battery research at the Mathematical Institute is conducted in collaboration with engineering groups across Oxford. The University of Oxford is a founding partner of the Faraday Institution – the UK’s independent institute for electrochemical energy storage research. This partnership has allowed us to develop exciting research links with a number of Universities and industrial bodies across the UK. We also benefit from a number of industrial links, working with national and international partners BBOX, Nexeon and Siemens.
For more information about battery research at Oxford and its partners please visit the following links:
Oxford Mathematics Battery Modelling
Oxford Research Software Engineering