Cancer is a complex and resilient set of diseases and the search for a cure requires a multi-strategic approach. Oxford Mathematicians Lucy Hutchinson, Eamonn Gaffney, Philip Maini and Helen Byrne and Jonathan Wagg and Alex Phipps from Roche have addressed this challenge by focusing on the mathematical modelling of blood vessel growth in cancer tumours.
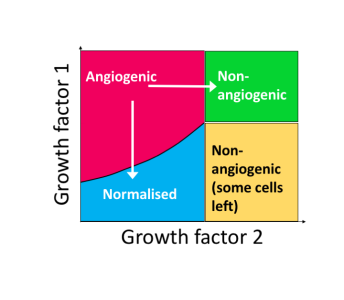
Angiogenesis, the formation of new blood vessels from existing ones, is a key characteristic of tumour progression. The purpose of anti-angiogenic (AA) cancer therapies is to disrupt the tumour’s blood supply, inhibiting delivery of oxygen and nutrients. However, such therapies have demonstrated limited benefit to patients; they do not consistently improve survival. It has been suggested that normalisation, the process by which vessels transition from the leaky state that is typical of tumour vasculature (the arrangement of the vessels) to a state where blood perfusion is increased, is the reason why some AA therapies lack efficacy.
Lucy and her colleagues have developed a mathematical model that accounts for the biochemistry of angiogenesis and vessel normalisation. They have shown that the model exhibits four possible long term behaviour regimes that could correspond to vascular phenotypes (an organism's observable traits) in patients. By identifying parameters in the mathematical model that lead to transitions between these behaviour regimes, the model can be used to determine which biological perturbations would theoretically lead to a transition between a given pre-treatment phenotype and the goal post-treatment phenotype. The implication of these mathematical models is that it enables 'personalised' therapy, where the normalisation level of a tumour's vasculature is taken into account when selecting the most beneficial type of anti-angiogenic treatment for a given tumour.
The research can be examined in more detail in the Journal of Theoretical Biology.