Statistical mechanics (or thermodynamics) is a way of understanding large systems of interacting objects, such as particles in fluids and gases, chemicals in solution, or people meandering through a crowded street. Large macroscopic systems require prohibitively large systems of equations, and so equilibrium thermodynamics gives us a way to average out all of these details and understand the typical behaviour of the large scale system. Typical quantities of liquid, for instance, contain more than $10^{23}$ molecules, and this is far too many equations for modern supercomputers, or even Oxford students, to handle. This theory of averaging, which has been refined and extended for over a century, gives us a way to take equations modelling individuals, and derive equations governing bulk behaviour which are fundamentally easier to analyze. But it is not applicable to so-called fluctuating or nonequilibrium systems, where a net flow of mass or energy keeps the system away from equilibrium. Such systems are increasingly of interest to modern day scientists and engineers. Essentially all biological systems, for instance, are kept away from thermodynamic equilibrium, which in biology is also known as death.
Unfortunately, while mathematicians and physicists have struggled for many years to develop a theory capable of understanding nonequilibrium systems in general, many fundamental problems remain, and there is no consensus in the community about what approaches to take. This has led to several non-equivalent formulations which can give different predictions of physical phenomena, and far away from thermodynamic equilibrium these theories become increasingly difficult to reconcile. Near to equilibrium, on the other hand, most of these theories all become compatible with Linear Nonequilibrium Thermodynamics (LNET), which is attained in the limit of small (but nonzero) fluxes. Essentially this is just applying Taylor's Theorem from calculus: equilibrium thermodynamics is the first term in an expansion of most plausible theories of thermodynamics, and LNET is the first contribution of fluctuations to the system.
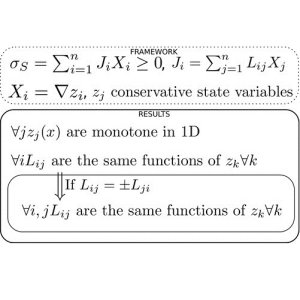
LNET can be characterised by an entropy production involving a matrix of "phenomenological coefficients," $L_{ij}$, relating thermodynamic fluxes and forces and which can often be determined experimentally. These coefficients give great insight into how to build a consistent macroscopic description of many nonequilibrium systems, such as the thermochemistry in Lithium-Ion batteries, or the dynamics of protein folding. While LNET has proven useful in many such applications, it is still far from a complete theory, especially regarding the coupling of different physical processes. An important tool for understanding such coupled processes are the Onsager-Casimir Reciprocal Relations, which essentially state that the phenomenological matrix described above has to have a certain symmetry property, so that (for state variables which are not changed by time reversal), $L_{ij}=L_{ji}$. These relations are still hotly contested in the community, though they have proven to be very powerful and seemingly consistent with many physical systems. These relations are useful both in terms of constraining plausible models, as well as in making the determination of these coefficients easier (as one would only need to find a subset of them experimentally in order to construct the entire matrix).
Contemporary nonequilibrium thermodynamics has become heavily invested in studying multiple coupled processes, and in this case the matrix $L_{ij}$ plays a central role in the theory. These coupled processes are of utmost interest as they bring insight into otherwise perhaps non-intuitive phenomena. For example, one chemical reaction can run against its natural direction (negative Gibbs energy) by using a positive source of entropy from another process, such as heat flow or another chemical reaction. These ideas are behind explanations of the classical experiment of Duncan-Tor which is outside of the classical Fickian concept of diffusion but fits closely with the Maxwell-Stefan model (which essentially is the coupled analogue of Fick's diffusion law). Further illustrations include the important electrophoretic drag in PE fuel cell membranes, thermodiffusion coupling (Soret's effect), which has been used in isotope separation in the Manhattan project, and similarly Seebeck's and Peltier's thermoelectric effects, which have found many applications. All of these phenomena are surprising in that they run against classical intuition, and cannot be explained by equilibrium thermodynamics.
Recently, Vaclav Klika from the Czech Technical University in Prague, and Oxford Mathematician Andrew Krause, have further contributed to our understanding of LNET by finding functional constraints that these coefficients must satisfy. The research, published in the Journal of Physical Chemistry Letters, shows that any dependence of these coefficients on state variables (such as temperature) must be shared among some or all of the phenomenological coefficients, and they cannot vary independently of one another. Additionally, while the Onsager-Casimir relations need certain assumptions to derive which are presently debated in the community, some version of these functional constraints must hold in general systems with conservative state variables. If the Onsager-Casimir relations are applicable to a system, then these functional constraints are even more powerful, showing that any dependence the phenomenological coefficients have on state variables must be the same for the whole matrix, and hence can be determined much more easily through experiments. More provocatively, these constraints suggest that many well-studied models in the literature have employed constitutive relations which are not thermodynamically consistent, and so would need to be revisited in light of these results.
While there is still a tremendous amount of work left to do in extending these tools more generally, this research has shown the power of mathematics in the physical and life sciences, and will hopefully prove a useful step toward developing a more complete understanding of nonequilibrium systems.