The virus causing the COVID-19 pandemic, SARS-CoV-2, is transmitted through virus-carrying respiratory droplets, which are released when an infected person coughs, sneezes, talks or breathes. Most of these droplets will fall to the ground within two metres, hence the guidelines to maintain social distancing. However, some droplets are small enough to float in the air. These droplets may remain airborne for hours and be carried throughout a room, leading to airborne transmission.
Models studying the infection risk from airborne transmission generally fall into one of two types. The first are Computational Fluid Dynamics (CFD) models, which can take into account the many complex variables such as the room size and geometry, the complex turbulent airflow and the size distribution of the aerosols. However, such models require specialised software and are computationally demanding, taking a long time to run even for small rooms. Hence, CFD models cannot be easily applied to new rooms and are not suited for rapid simulations, which are essential in a fast-evolving pandemic.
The second method to determine the infection risk is the Wells–Riley model. This model assumes that the air in the room is well-mixed, so the airborne infectious droplets are instantaneously evenly distributed throughout the room, and that they are removed by ventilation and gravity uniformly throughout the room. This assumption implies that everyone in the room is equally likely to be infected, which, in many cases, is not true. However, Wells–Riley models continue to be frequently used to inform pandemic predictions as they are easy to implement and very fast to run. Oxford Mathematician Ian Griffiths worked with Katerina Kaouri, Aaron English and Zechariah Lau from Cardiff University, in collaboration with the Welsh Government, to develop a model that bridges the gap between CFD models and Wells–Riley models. Their model is an extension to the Wells–Riley model that allows fast predictions of how the infection risk varies across the room as time evolves.
The model consists of an advection–diffusion–reaction equation, which governs the concentration of the airborne infectious droplets in the room. This equation describes the effects of the virus-carrying droplets being advected by the airflow, diffused due to turbulence, and emitted by infected people as well as being removed due to ventilation, inactivation of the virus and gravitational settling as in the Wells–Riley model.
The results of the model agree well with three real case studies: a courtroom, a restaurant and a hospital ward. Just as importantly, the model takes only a few minutes to run on a laptop computer. The work performed by the team has therefore addressed the need for a quick-to-run and efficient model that determines the spatially dependent airborne infection risk of COVID-19. In particular, when new variants of the virus are discovered, the model can quickly be updated in order to make policy recommendations.
Most recently, the team, in collaboration with industrial partners, have been exploring how air-purifiers can help mitigate the spread of COVID-19 in indoor spaces such as schools and restaurants. They aim to provide a strategy and policy recommendations for the optimal placement of air-purifiers to mimimise the infection risk in an indoor space. This strategy will be an important tool in our arsenal against the pandemic as we try to move forward to a ‘new normal’.
This work has been funded by the Welsh Government, the Royal Society and Cardiff University, and involved important discussions with Raquel González Fariña, Attila Kovács and Xander Pretty.
For more information, you can read the journal article here.
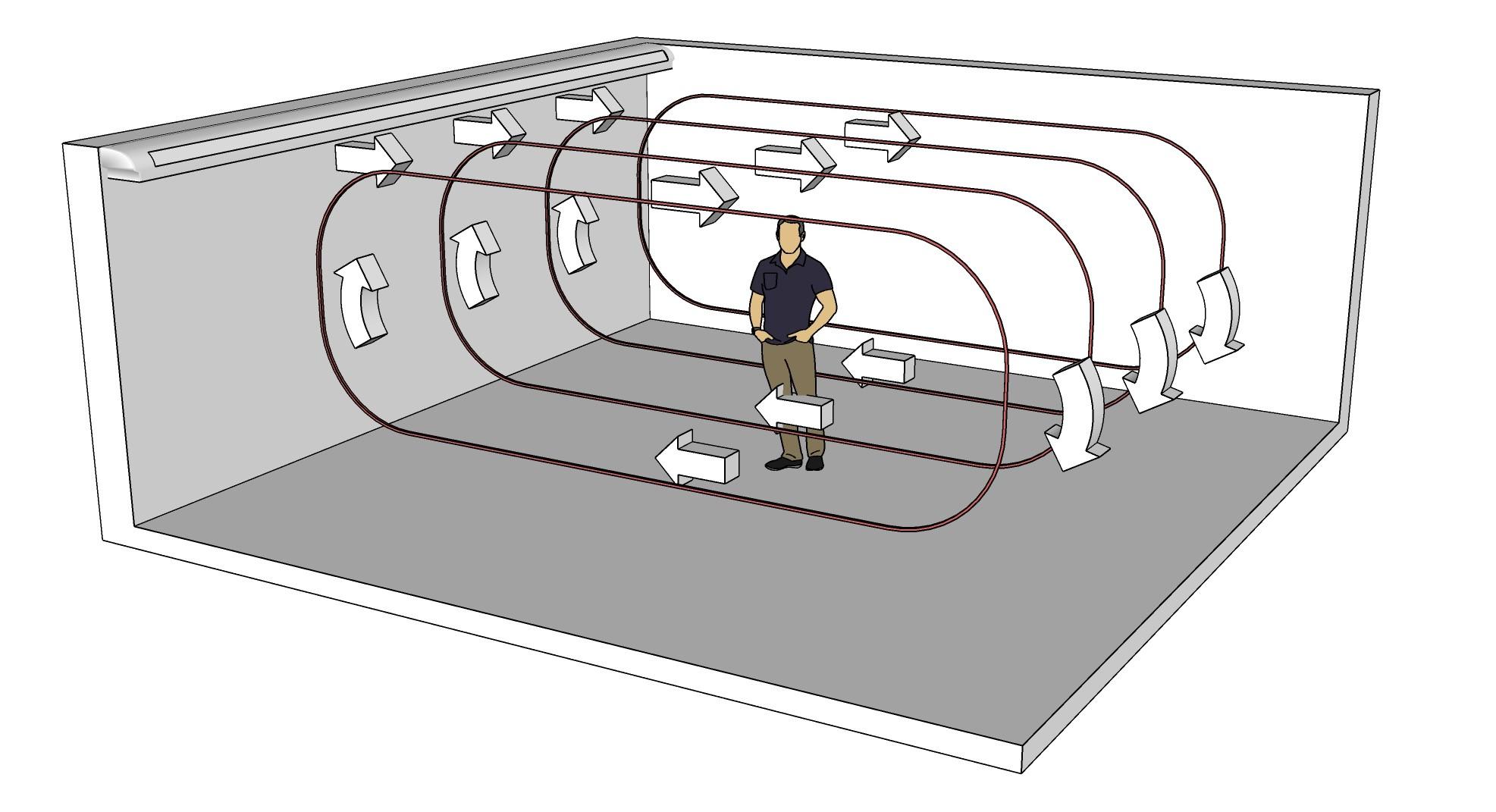
Figure: Schematic of a room configuration illustrating air recirculation generated by an air-conditioning unit.


