As cancer treatment improves and survival rates increase, the long- and short-term side-effects of these treatments become more of a concern. One such side-effect is autoimmune myocarditis, or cardiac muscle inflammation, which can occur in patients undergoing treatment with immune checkpoint inhibitors (ICIs), a class of drugs used in cancer therapy. Although autoimmune myocarditis is a rare side-effect affecting only 0.1-1% of patients being treated with ICIs, it has a high fatality rate at 25-50% of cases. Despite extensive experimental work, much remains unknown about the immunological pathways that drive the development and progression of this disease, and how those processes are influenced by the presence of ICIs. Furthermore, no pre-clinical in vitro assay currently exists that can screen potential new drug compounds for cardiotoxicity, so serious cardiac side-effects like autoimmune myocarditis are only discovered once they occur in patients in clinical trials or thereafter [1].
Mathematical modelling is an ideal tool to improve our understanding of the immunology underlying autoimmune myocarditis, and aid in the design of an in vitro cardiotoxicity assay. We have built the first mathematical model of autoimmune myocarditis based on the immunological pathways implicated as important in the biological literature [2], and simplified it to obtain the minimal set of ordinary differential equations that can reproduce the disease characteristics. Fig. 1 shows a graph representing the variables and interactions included in the model.

Figure 1: Graphical representation of the mathematical model of autoimmune myocarditis and the effects of the immune checkpoint inhibitors nivolumab ($D_N$) and ipilimumab ($D_I$). Dead/damaged cardiomyocytes are denoted $C$, innate immune cells are denoted $I$, pathogenic CD4+ T cells are denoted $P$ and regulatory T cells are denoted $R$. Arrow heads indicate stimulation and bar heads indicate inhibition.
In addition to using the insights gained from this model to design an in vitro cardiotoxicity assay, we can also explore the possibility of scheduling ICI treatment to minimize the risk of autoimmune myocarditis developing. To do this, we need to connect our model of autoimmune myocarditis to a model of tumour growth and track both the positive and negative effects of the ICI therapy. A standard-of-care treatment schedule of combination therapy, in which both nivolumab and ipilimumab are administered, delays tumour growth but also causes autoimmune myocarditis to develop (see left-hand column in Fig. 2). We can use optimal control theory to optimize the timing of the ICI doses such that tumour growth is still delayed, but autoimmune myocarditis no longer develops (see right-hand column in Fig. 2). Notably, this optimized dosing schedule differs significantly from the current standard-of-care schedule!
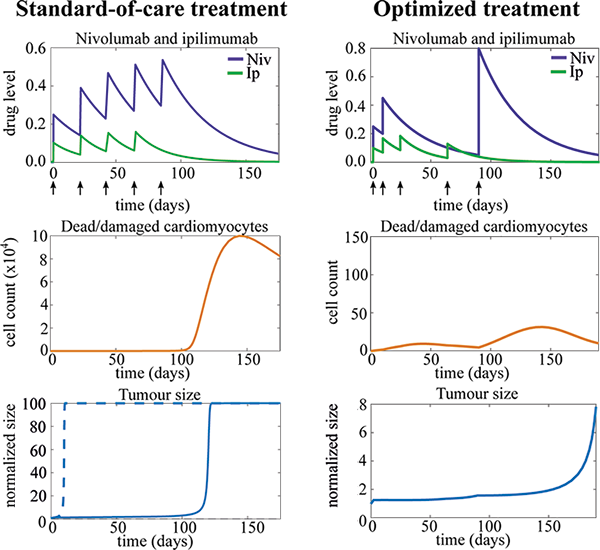
Figure 2: Plots of ICI therapy (top), dead/damaged cardiomyocytes (middle) and tumour size (bottom) over time when a standard-of-care (left) or optimized (right) treatment schedule is applied. The optimized treatment schedule still delays tumour growth, but now tissue damage (represented by the dead/damaged cardiomyocytes) remains low, meaning autoimmune myocarditis no longer develops. Tumour size has been normalized by initial size. The dashed line indicates tumour growth without any therapy. Arrows indicate time points at which one or multiple doses of ICIs are administered.
When deciding how to schedule cancer treatments, it is important to consider both the desired and undesired effects of treatment. Current cancer therapy modelling often only includes the effects of therapy on the tumour but does not explicitly consider potential side-effects of the treatment, as we have done here. Thus, we advocate for a more holistic approach to cancer therapy modelling where we consider the effect of a specific therapy in a number of different connected compartments, which represent both the tumour and specific tissues or organs in the body. In this way we can consider effectiveness and safety of the therapy simultaneously.
Solveig van der Vegt is a postgraduate student in Oxford Mathematics.
References:
[1] S. A. van der Vegt, Y.-J. Wang, L. Polonchuk, K. Wang, S. L. Waters, and R. E. Baker. A new approach in immune checkpoint inhibitor-induced autoimmune myocarditis research (preprint).
[2] S. A. van der Vegt, L. Polonchuk, K. Wang, S. L. Waters, and R. E. Baker. Mathematical modelling of autoimmune myocarditis and the effects of immune checkpoint inhibitors. Journal of Theoretical Biology, 537:111002, 2022.


