A new study uncovers a surprising connection between sliding droplets and migrating groups of cells. By combining live 3D imaging with mathematical modelling, scientists have revealed how cell collectives organise themselves in space and time, which depends on the cell collectives behaving like fluids. Just like raindrops racing down a window pane, the cells are guided by forces like surface tension and viscosity, allowing them to move as a coordinated unit while they respond to chemical signals.
From immune cells to flocks of birds to even human crowds, collective directed movement is everywhere in life. Yet, there are still many mysteries around how groups of cells coordinate their motion, especially when navigating complex environments, such as human tissue or soil. Such collective migration is often guided by chemical cues, a process referred to as collective chemotaxis, which organises several physiological processes, such as tissue regeneration and embryo development, but also diseases, such as cancer.
Mathematicians have long been intrigued by the mechanisms behind collective chemotaxis. One of the foundational contributions to this field came from Keller and Segel, who developed the first mathematical model describing how cells migrate up a self-generated gradient of chemical attractants. Their model, which is expressed as a system of coupled partial differential equations, elegantly captures how cells and chemical signals interact across space and time. However, there is a catch.
In the classical Keller–Segel model, physical interactions between cells are neglected. As a result, cells can be treated like biased gas molecules, which independently respond to chemical cues. This simplification works surprisingly well in low-density populations, where cells move relatively freely. However, in many biologically relevant situations, such as during embryonic development, wound healing, tumour invasion, or emperor penguins in the Antarctic, cells organise and migrate in densely packed groups.
In a recent study published in the journal PNAS, an interdisciplinary team of mathematicians and developmental biologists from the Universities of Oxford and University College London explored the material properties of migrating cell populations. Their study reveals how physical interactions among densely packed cells give rise to emergent fluid-like properties, including surface tension and viscosity. This results in a novel theoretical picture of chemotaxis in which migrating cell populations behave as a growing, chemotaxing thin fluid film.
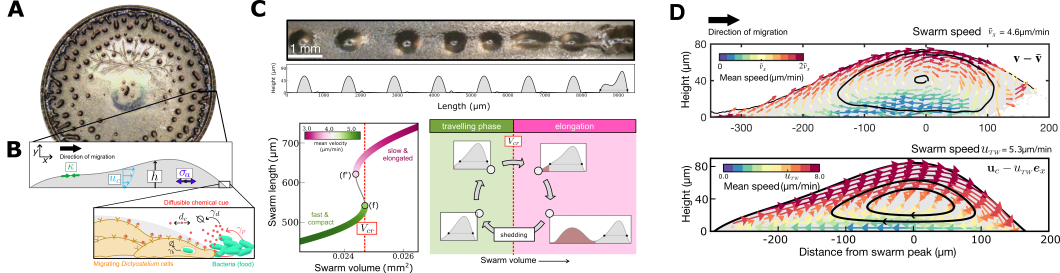
Figure caption: (A) Snapshot of pattern formation during feeding front expansion of Dictyostelium colonies. (B) Schematic summarising the key features of the active fluid model for the directed migration of cell groups. (C) The model recapitulates the experimentally observed shedding pattern and links it to a transition in travelling wave solutions – a specific solution to the problem. (D) Validation of the predicted cell flow patterns from the active fluid model.
By combining theoretical predictions with experimental observations, the group uncovered surprising consequences of this fluid-like behaviour. Within migrating groups, cells form well-defined flow patterns, similar to how fluid moves within a sliding droplet. Even more strikingly, the collective migration periodically produces shedding events driven by a sudden transition in the shape - or morphology - of the migrating group. These shedding events release clumps of cells from the rear of the group, which go on to form environmentally resistant spores (see movie). Crucially, which cells get shed is influenced by the internal flow dynamics of the group. This suggests that the group’s emergent material properties are not just by-products of migration, but active regulators of biological function.
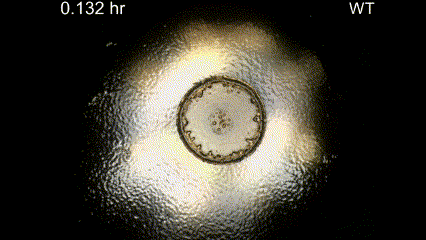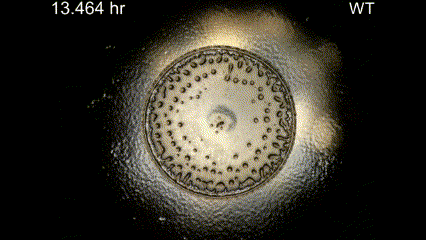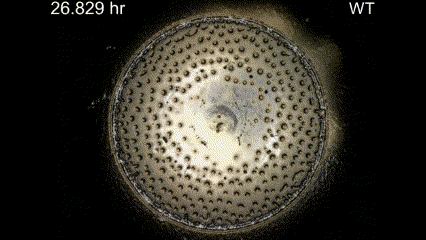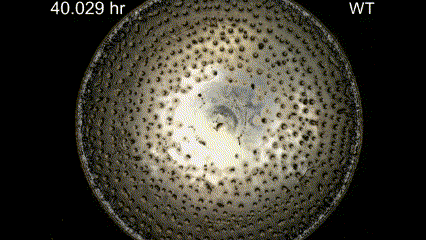
Movie Caption: Macrophotography of pattern formation during feeding front expansion of Dictyostelium colonies.
“As a kid, I was captivated by raindrops running down the car window during long trips. I’d watch them zigzag and merge, racing to the bottom, trying to guess which one would reach the edge first. Little structure was hidden behind what appeared random. Years later, as a mathematical biologist, I never imagined I’d find myself once again mesmerised by moving droplets. But this time, they weren’t made of water - they were groups of living cells. And mathematics became the key to disentangling the hidden rules behind their behaviour,” said Dr Giulia Celora, a Hooke Research Fellow at the Mathematical Institute.
By using advanced imaging techniques and the theoretical framework of biological fluids, this work offers a new perspective on the study of chemotaxis of multicellular communities, revealing the role of physical interactions in mediating their collective dynamics. Since collective migration is a common feature across biological systems - from embryonic tissues to invasive tumours - these findings have broad implications for our understanding of developmental biology, disease progression, and tissue organisation. This study also opens up exciting new avenues for mathematical exploration of the principles and transitions regulating the organisation of biological living matter.
The team working on the research comprises Giulia Laura Celora (author of this piece and pictured), Hugh Ford, Elizabeth Westbrook, Hella Baumann, Cornelis Weijer, Benjamin Walker, Mohit Dalwadi, Philip Pearce and Jonathan Chubb.


