Tissue oxygenation plays a crucial role in the growth of cancerous tumours and their response to treatments. While it may seem intuitive that reducing oxygen delivery to a tumour would be a treatment therapy, low oxygen levels (hypoxia) can significantly reduce the effectiveness of treatments such as radiotherapy and some chemotherapies. Therefore, understanding the dynamics of a tumour's red blood cells - which carry oxygen through the vasculature - is of vital importance. However, it is a very challenging problem, as tumour vasculature is highly irregular spatially, and can change rapidly over time.
This problem has been the focus of a multi-disciplinary investigation, led by Miguel Bernabeu from the Centre for Medical Informatics, University of Edinburgh, Tomas Alarcon, CRM, Barcelona (both formerly at Oxford) and Oxford Mathematician Helen Byrne; and also involving Oxford colleagues Philip Maini (Mathematics), Joe Pitt-Francis (Computer Science) and Ruth Muschel (Oncology), together with researchers from Ljubljana, the Alan Turing Institute and University College, London.
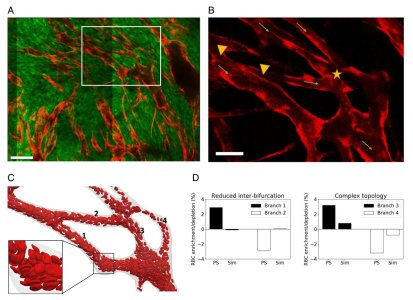
In a recently published paper in the Proceedings of the National Academy of Sciences they have analysed in detail how red blood cells distribute throughout tumour vasculature. Analysis of vascular networks in mouse tumours revealed abnormal morphological patterns, including reduced inter-bifurcation distance and branching points with topological configurations different from the typical diverging/converging bifurcations. A computational model showed that such a branching network disrupted the flow of red blood cells in a way that was enhanced as the flow went through subsequent bifurcations. This “memory” effect led to the derivation of a new rule for how haematocrit, or the volume of red blood cells, splits at bifurcations and the emergence of a new metric (mean vessel length-to-diameter ratio), as a predictor for tissue oxygen heterogeneity.
The paper finds that the mean vessel length-to-diameter ratio increases in tumours that have received anti-angiogenesis treatment, and predicts that this would lead to less heterogeneity in haematocrit distribution and, hence, a more spatially uniform oxygen profile within the tumour. It is therefore proposed that this metric could be used to monitor the action of anti-angiogenic agents. Moreover, it makes a significant step in unravelling the causal relationship between tumour vascular structure and tissue oxygenation which, in turn, has the potential to pave the way for new personalised therapeutic approaches.
Figure above: analysis of images of tumour vasculature in mice (A, B) allows us to extract realistic geometries on which to carry out simulations of the mathematical model (C), enabling us to collect in silico data on red blood cell (RBC) flow and to calculate how the topology of the network affects RBC distributions in individual vessel branches (D).