Modelling Removal of Sulphur Dioxide from Flue Gas
- Researcher: Kristian Kiradjiev

- Academic Supervisors: Chris Breward, Ian Griffiths and Donald Schwendeman (Rensselaer Polytechnic Institute)
- Industrial Supervisors: Uwe Beuscher, Vasudevan Venkateshwaran
Background
In order to remove sulphur dioxide from a stream of flue gas, a purification device containing special thin porous sheets can be used. Gas containing sulphur dioxide is passed down the outside of the sheets and enters the pores by diffusion. Inside the pores, there are microscopic pellets held together by a network of fibres, where a chemical reaction turns gaseous sulphur dioxide into liquid sulphuric acid. The accumulation of liquid limits the transport of sulphur dioxide to the reaction surface resulting in a loss of removal efficiency. This project aims to understand the effect of key material and operational parameters on the creation, motion, and accumulation of the liquid sulphuric acid within the porous medium.
Figure 1: Three modules of Gore's device.
Outcomes
We started exploring the problem on the microscale around a single catalytic pellet. We analysed how liquid is generated around it and how it spreads along fibres that are connected to the pellet [1]. We then derived a model that upscales the local complex behaviour of the microstructure of the filter sheets to a device-scale model that describes how the liquid sulphuric acid and the gaseous sulphur dioxide are transported within the filter [3].
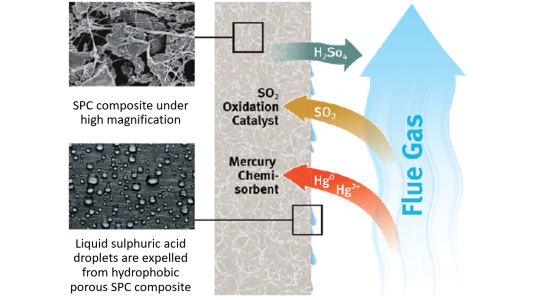
Figure 2: Schematic diagram of the operating mechanism of the purification device.
In the plot below, we show a temporal profile of the sulphur dioxide concentration at the outlet of the filter channels for different gas speeds.
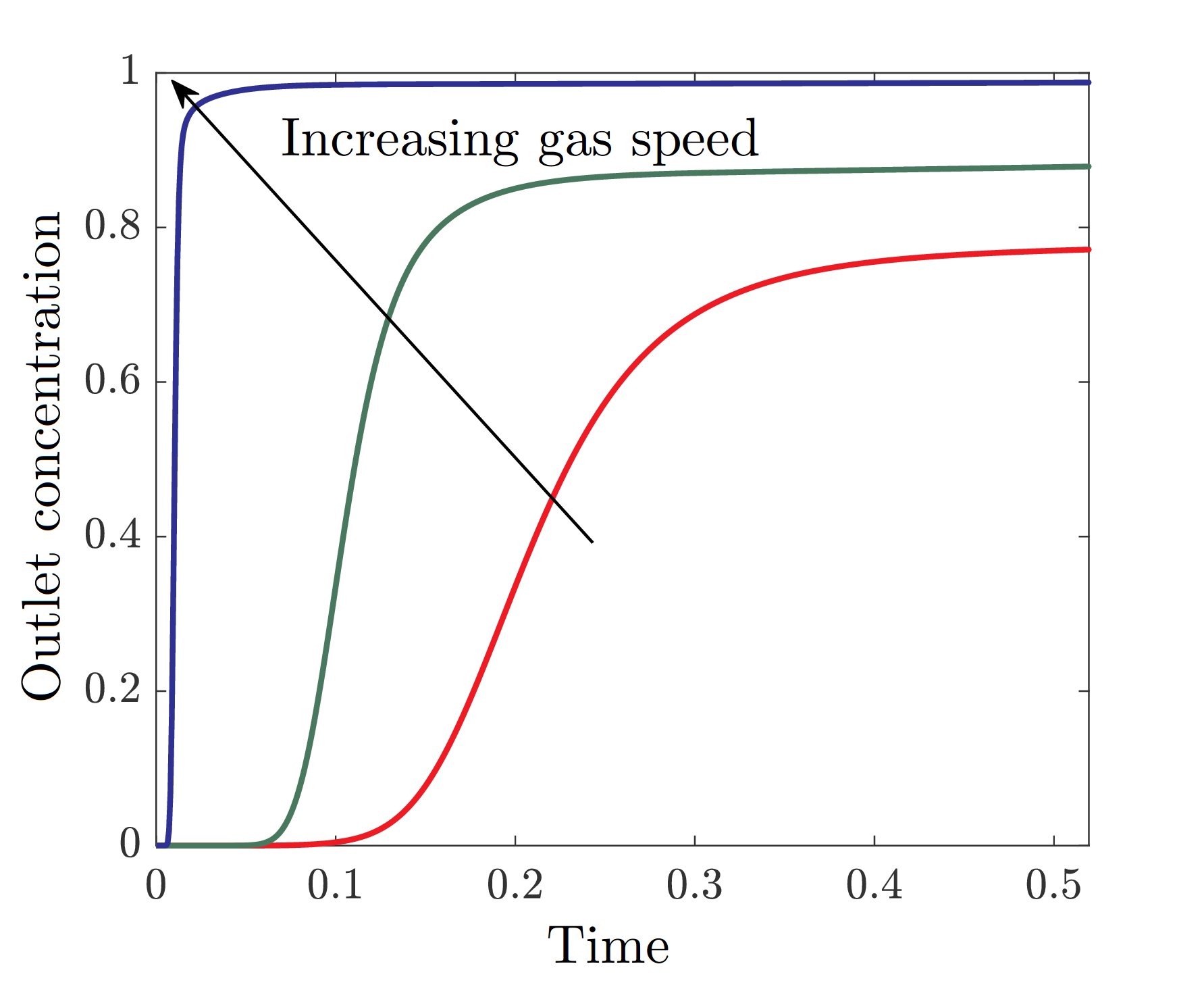
Figure 3: Temporal profile of the concentration of sulphur dioxide at the outlet of the filter channels for different gas speeds, where both time and outlet concentration are normalised.
We see that, increasing the gas speed increases the concentration of sulphur dioxide, since there is less residence time for the gas in the filter. Thus, if the concentration cannot exceed a given threshold, we can calculate the maximum speed the gas can be flowed at to achieve this goal. Since sulphuric acid is hygroscopic (has high affinity to water), we also developed a separate model that describes this behaviour [2]. The results were validated through a set of experiments performed at Gore. We developed a model for a filter made of hydrophilic fibres as well, which floods more uniformly with liquid.
Publications
[1] K. B. Kiradjiev, C. J. W. Breward, I. M. Griffiths. Surface-tension-and injection-driven spreading of a thin viscous film. J. Fluid Mech., 2019.
[2] K. B. Kiradjiev, V. Nikolakis, I. M. Griffiths, U. Beuscher, V. Venkateshwaran, C. J. W. Breward. A Simple Model for the Hygroscopy of Sulfuric acid. Ind. Eng. Chem. Res., 2020.
[3] K. B. Kiradjiev, C. J. W. Breward, I. M. Griffiths, D. W. Schwendeman. A Homogenised Model for a Reactive Filter. (Under Review), 2020.

